Give us a call:
+91-22-41611111
+91-22-41611111
Send us a mail:
indeus@indeus.in
indeus@indeus.in
Come visit us:
Directions to our location
Directions to our location
We offer our clients high quality formulation development services for the regulated markets in the US and the European Union (region). The facility houses a state-of-the art formulation development laboratory, an analytical laboratory and a cGMP certified pilot plant.


We offer our clients high quality formulation development services for the regulated markets in the US and European Union. We have a state-of-the art facility with formulation development laboratory, an analytical laboratory and a cGMP certified pilot plant.
We have invested in a state of art facility which has EU-GMP accreditation for manufacturing tablets, Formulation and Analytical R & D for development of predominantly solid orals.
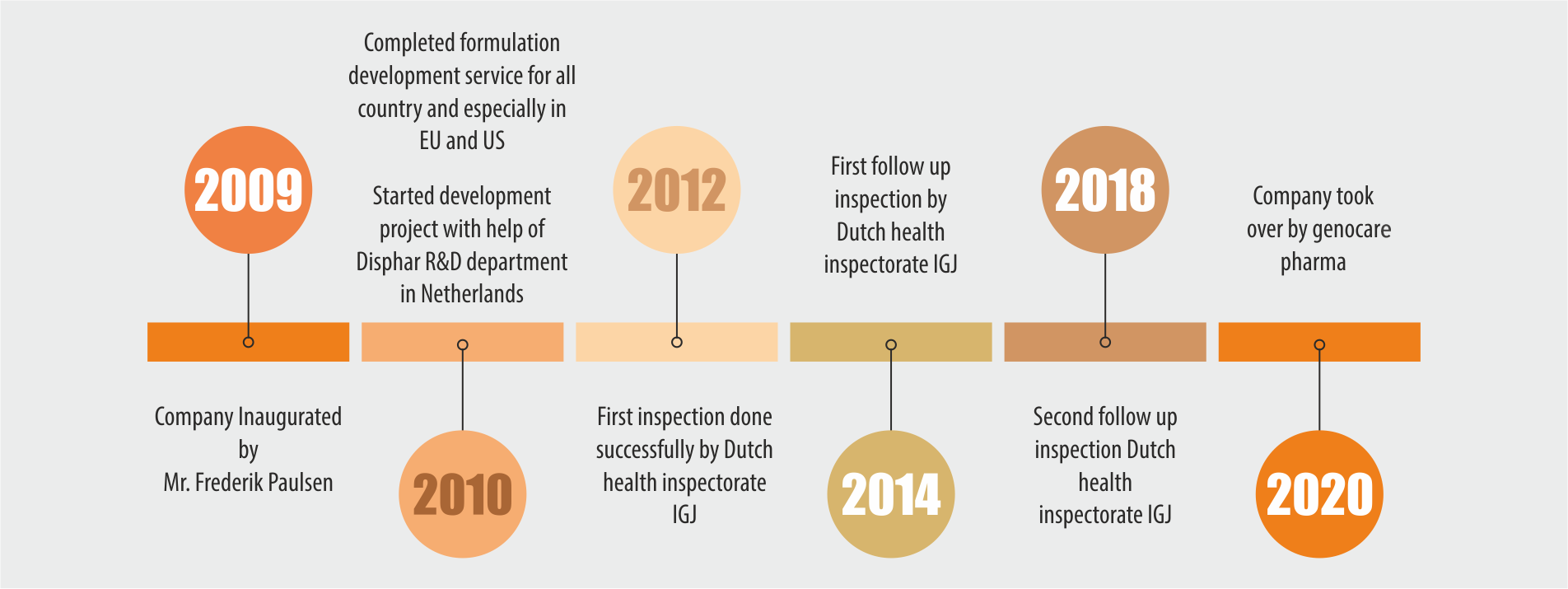
Nordic BV and Disphar international Set up ‘Indeus Life Sciences Pvt. Ltd.’ India, to develop generic drugs and either perform tech transfer to a selected site or manufacturer commercial batches for clients in EU. Which was acquired by Indian technocrats (Genocare Pharma) along with IPS.
The Pharmacist with a Master’s Degree and 12 years of experience as Head of R&D Formulation, Regulatory Affairs, and Project Management of niche dosage forms for international markets has an exceptional background in the pharmaceutical industry. Having worked at renowned pharmaceutical companies such as Kopran, Unichem, Merind, Sigma, SM Pharmaceuticals, and Asia Pharmaceuticals, this individual has demonstrated expertise in developing both conventional and non-conventional drugs for global markets.
He has extensive experience in regulatory affairs, ensuring compliance with local and international regulations and guidelines.
He has a Master’s Degree and extensive experience as Head of R&D Formulation, Regulatory Affairs, and Project Management brings a wealth of expertise in niche dosage forms for international markets. Their skills and accomplishments position them as a valuable asset in the pharmaceutical industry, particularly in the areas of formulation development, regulatory compliance, and project leadership.
Samir is an experienced professional with over 18 years of expertise in the Pharmaceutical International Business/Export-Import, Pharma Management, Contract Manufacturing, and New Product Development. He is not only a passionate entrepreneur in the pharmaceutical industry but also has interests in other priority sectors.
Specialties in :
Business Development, In-Licensing, Out-Licensing, Merger & Acquisitions, Market Analysis & Market Intelligence, Medical Evaluation, Product Selection, Portfolio – Pipeline Management.
Samir’s extensive experience and specialties in business development, in-licensing and out-licensing, mergers and acquisitions, market analysis, market intelligence, medical evaluation, and product selection make him a valuable professional in the pharmaceutical industry. His entrepreneurial drive and involvement in various pharmaceutical companies further demonstrate his dedication to driving growth and success in the sector.
Lead pharmacist Finny Joseph is a registered pharmacist; Since 1997, Finny has been servicing patients and health care providers in hospitals and pharmacies.
Finny’s goal is to be a collaborative partner providing the education that leads to improved adherence and therapy outcomes.He believes in the power of education and strives to provide patients with the necessary information and guidance to make informed decisions about their healthcare. Under his guidance, Josefs Pharmacy has a consistently growing clientele who appreciate fast, reliable, service while knowing they have a trusted healthcare advisor.
In addition to his experience and patient-centered approach, Finny has also completed pharmaceutical compounding training at the University of Florida. This specialized training equips him with the skills and knowledge necessary to prepare customized medications tailored to the unique needs of individual patients.
A Pharmacist with a Ph.D. in cleaning Validation. Total experience of 28 years in Quality Control and Quality Assurance and running plant operations. Previously worked with J&J, Lupin & IVAX. Experience of facing Preapproval inspection of FDA. Present at Indeus since inception.
GM Operations- Msc
Head R&D- PhD
